

The Pfizer-BioNTech bivalent COVID-19 booster is authorized for use as a single booster dose in people 12 years old and up.Ī patient can choose to receive either the updated Pfizer booster or the updated Moderna booster, regardless of which company’s primary dose series or booster vaccine they received previously. Moderna’s bivalent COVID-19 booster is authorized for use as a single booster dose in people 18 years old and up. The bivalent vaccines, which offer better protection against COVID-19 caused by the omicron variant, have been authorized for use as a single booster dose at least two months after primary or booster vaccination.

Digital scheduling offers patients flexibility and convenience, as well as the ability to schedule multiple patients at once, which makes it easier for families and other groups that want to get vaccinated together. Since initial supply is limited, patients who would like to receive the updated boosters are encouraged to make an appointment using our digital scheduler. The government’s distribution of the updated boosters is underway and individual CVS Pharmacy locations are receiving the bivalent COVID-19 boosters on a rolling basis over the next few days and weeks.Īppointments are made available at CVS.com and via the CVS Pharmacy app as doses are received. Tobacco-Free Generation Campus InitiativeĬVS Pharmacy locations nationwide are offering the FDA- and CDC-authorized Pfizer-BioNTech and Moderna bivalent COVID-19 booster vaccines.Task Force on Climate-Related Financial Disclosures.Environmental, Social and Governance (ESG) report.Corporate social responsibility overview.The primary series of two-dose vaccines should still be completed with the same product for both doses.We celebrate Sue and all our amazing pharmacy technicians, on National Pharmacy Technician Day.However, if the original product is not available or another product is preferred, mix and matching vaccines with any of the authorized COVID-19 vaccine boosters is allowed.
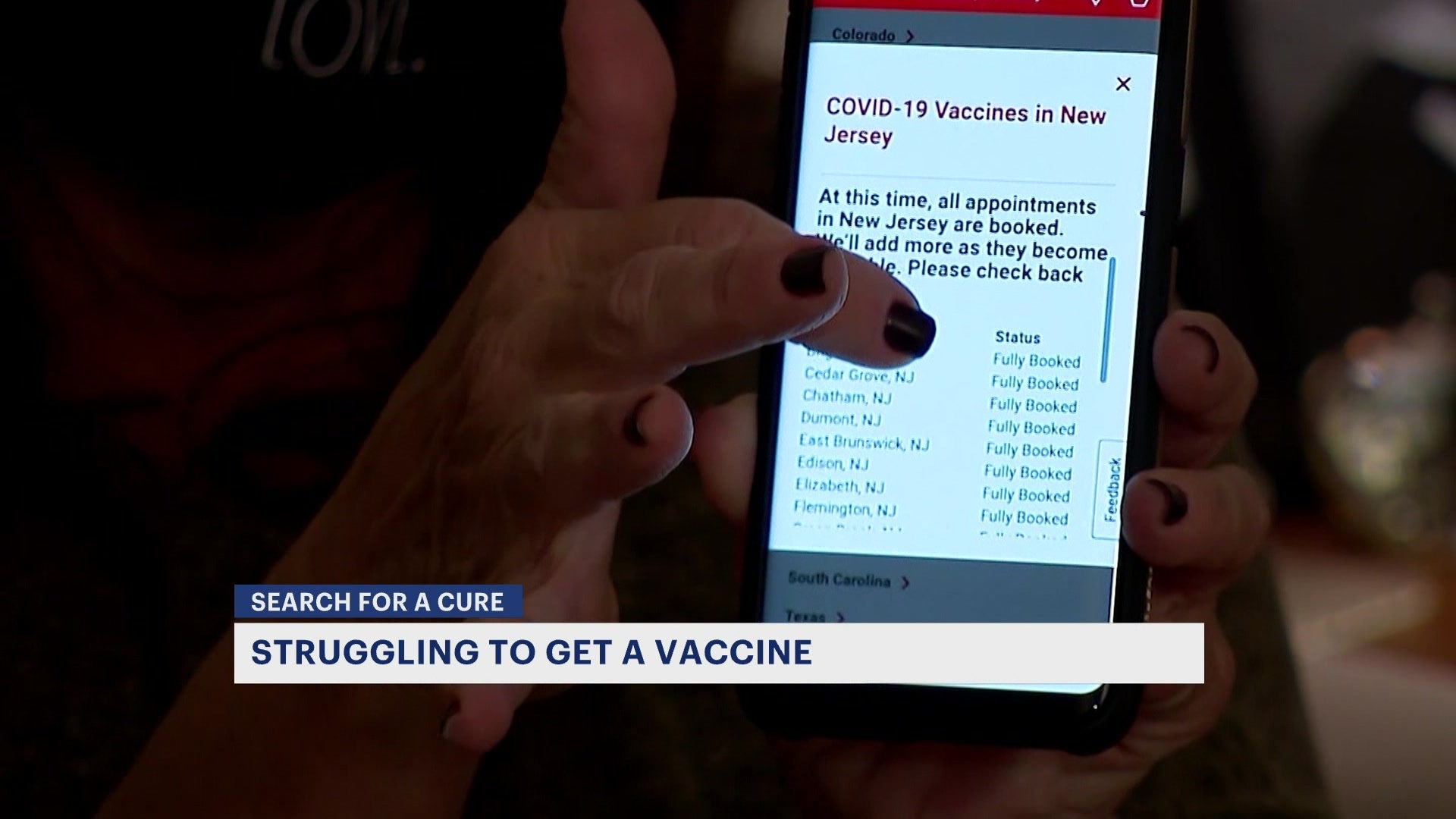 Mix & Match Guidance: In general , it is recommended individuals receive the same product for their booster dose as they did for their primary series. J&J COVID-19 Booster Eligibility: A single vaccine booster dose is recommended for individuals 18 and older who received a J&J primary dose at least 2 months after they receive their initial J&J primary vaccine dose. Individuals ages 18-64 who are at an increased risk for COVID-19 exposure and transmission because of occupational or institutional setting, such as healthcare and essential workers, based on their individual benefits and risks. Individuals ages 18 – 49 should consider individual benefits and risks, according to CDC guidance. Individuals aged 18 and older with underlying medical conditions. Long-term care facility residents ages 18 and older. Individuals must wait at least six months after they complete their initial mRNA COVID-19 primary vaccine series to receive their booster dose. Moderna COVID-19 Booster Eligibility: Eligibility requirements for Moderna and Pfizer-BioTech booster doses are the same. In addition to previously-announced Pfizer vaccine booster shot eligibility, here's a look at who can now get the booster shots: Walgreens said that while "it is recommended individuals receive the same product for their booster dose as they did for their primary series," if the original brand is not available or another brand is preferred, mixing and matching will be allowed. “Walgreens pharmacy teams are available to answer questions and make it easy to understand eligibility requirements and access COVID-19 vaccine, whether it’s a first dose or booster shot,” Rina Shah, group vice president pharmacy operations and services at Walgreens, said in a statement. Suburban Comedy Club Auctioning Off Memorabilia to Pay Back Loansīoth pharmacy chains noted that eligible populations can now choose to receive a dose different from the one they received for their initial series, following new guidance from both the CDC and the Food and Drug Administration.
Mix & Match Guidance: In general , it is recommended individuals receive the same product for their booster dose as they did for their primary series. J&J COVID-19 Booster Eligibility: A single vaccine booster dose is recommended for individuals 18 and older who received a J&J primary dose at least 2 months after they receive their initial J&J primary vaccine dose. Individuals ages 18-64 who are at an increased risk for COVID-19 exposure and transmission because of occupational or institutional setting, such as healthcare and essential workers, based on their individual benefits and risks. Individuals ages 18 – 49 should consider individual benefits and risks, according to CDC guidance. Individuals aged 18 and older with underlying medical conditions. Long-term care facility residents ages 18 and older. Individuals must wait at least six months after they complete their initial mRNA COVID-19 primary vaccine series to receive their booster dose. Moderna COVID-19 Booster Eligibility: Eligibility requirements for Moderna and Pfizer-BioTech booster doses are the same. In addition to previously-announced Pfizer vaccine booster shot eligibility, here's a look at who can now get the booster shots: Walgreens said that while "it is recommended individuals receive the same product for their booster dose as they did for their primary series," if the original brand is not available or another brand is preferred, mixing and matching will be allowed. “Walgreens pharmacy teams are available to answer questions and make it easy to understand eligibility requirements and access COVID-19 vaccine, whether it’s a first dose or booster shot,” Rina Shah, group vice president pharmacy operations and services at Walgreens, said in a statement. Suburban Comedy Club Auctioning Off Memorabilia to Pay Back Loansīoth pharmacy chains noted that eligible populations can now choose to receive a dose different from the one they received for their initial series, following new guidance from both the CDC and the Food and Drug Administration.







 0 kommentar(er)
0 kommentar(er)
